Silicone O-rings for De Soutter EcoPulse™ lavage system
De Soutter Medical Ltd specialises in the development, production and worldwide distribution of high performance orthopaedic tools for surgical procedures, offering their customers a comprehensive range of technically innovative and high quality products. The company approached us to manufacture two different sized Silicone O-rings for the ECO Pulse upgraded design.
The application
De Soutter Medical tasked us to support them with the pioneering new EcoPulse™ lavage system for use in orthopaedic surgery. With the brief of needing two different sized O-rings for a clinical application, our material expertise was needed.
The EcoPulse™ connects onto the front of a reusable De Soutter handpiece. This allows surgeons to lavage the surgical site using saline water. It is then simultaneously connected to a suction device to remove waste from the surgical site. The EcoPulse™ is supplied as a single use sterile packed product. There are a range of nozzles available for specific surgical procedures.
The new EcoPulse™ has a pared back functional design to eliminate superfluous plastic. Therefore, instead of using disposable batteries (and associated single use wiring and motors) it connects onto a reusable power tool. This tool is already being used to perform the surgical procedure.
This eliminates a large amount of clinical and WEEE waste. Additionally, compared to other products in the market, reduces clinical waste by up to 60%.
Our sealing solution
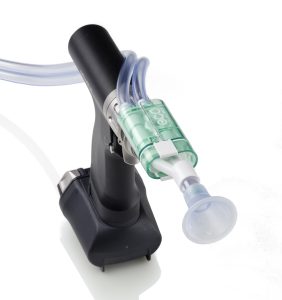
To ensure no saline leaks, the seal application is located within the disposable pump/irrigation attachments. Additionally, it is important there is no loss of suction during use.
The application was relatively straightforward in terms of mechanical sealing. However, due to the nature of the product there were critical demands on the material and during the manufacturing process.
For material selection our engineers specified a USP Class VI translucent silicone. This is suitable for medical devices due to its biocompatibility features and resistance to bacterial growth.
The use of release agents during the manufacture process of the O-rings is not necessary. This is due to the (bespoke and dedicated) moulding tool being coated in titanium nitride. Additionally, this is to mitigate the risk of any cross contamination with the O-rings. Eliminating risk is especially important when producing seals used within devices where there is any risk of patient fluids crossover.
Cleanroom manufacturing and the O-rings are double bagged in packaging to avoid any cross contamination. These parts are manufactured and packaged at our ISO13485 approved manufacturing site to achieve these requirements.
Customer satisfaction
Working in conjunction with our valued customer De Soutter Medical, we assist with design support and technical recommendations on the development and manufacture of sealing products for use within their medical devices.
For more on our full range of O-rings see this page: HERE
Read more about our Life Sciences & Medical expertise: HERE