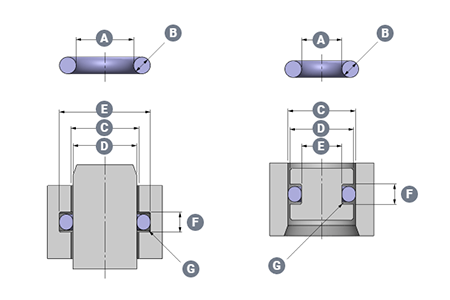
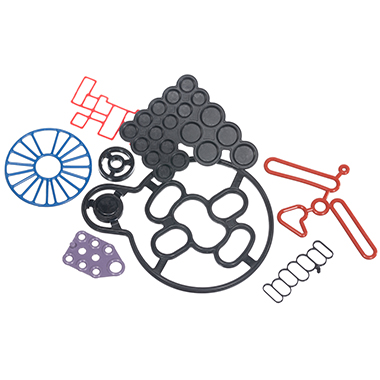
Silicone O-rings for De Soutter EcoPulse™ lavage systemSilicone O-rings for De Soutter EcoPulse™ lavage system De Soutter Medical Ltd specialises in the development, production and worldwide distribution of high performance orthopaedic tools for surgical procedures, offering their customers a comprehensive range of technically innovative and high quality products. The company approached us to manufacture two different sized Silicone O-rings for the ECO Pulse upgraded design. The application De Soutter Medical tasked us to support them with the pioneering new EcoPulse™ lavage system for use in orthopaedic surgery. With the brief of needing two different sized O-rings for a clinical application, our material expertise was needed. The EcoPulse™ connects onto the front of a reusable De Soutter handpiece. This allows surgeons to lavage the surgical site using saline water. It is then simultaneously connected to a suction device to remove waste from the surgical site. The EcoPulse™ is supplied as a single use sterile packed product. There are a range of nozzles available for specific surgical procedures.The new EcoPulse™ has a pared back functional design to eliminate superfluous plastic. Therefore, instead of using disposable batteries (and associated single use wiring and motors) it connects onto a reusable power tool. This tool is already being used to perform the surgical procedure.This eliminates a large amount of clinical and WEEE waste. Additionally, compared to other products in the market, reduces clinical waste by up to 60%. Our sealing solution To ensure no saline leaks, the seal application is located within the disposable pump/irrigation attachments. Additionally, it is important there is no loss of suction during use.The application was relatively straightforward in terms of mechanical sealing. However, due to the nature of the product there were critical demands on the material and during the manufacturing process.For material selection our engineers specified a USP Class VI translucent silicone. This is suitable for medical devices due to its biocompatibility features and resistance to bacterial growth.The use of release agents during the manufacture process of the O-rings is not necessary. This is due to the (bespoke and dedicated) moulding tool being coated in titanium nitride. Additionally, this is to mitigate the risk of any cross contamination with the O-rings. Eliminating risk is especially important when producing seals used within devices where there is any risk of patient fluids crossover.Cleanroom manufacturing and the O-rings are double bagged in packaging to avoid any cross contamination. These parts are manufactured and packaged at our ISO13485 approved manufacturing site to achieve these requirements. Customer satisfaction Working in conjunction with our valued customer De Soutter Medical, we assist with design support and technical recommendations on the development and manufacture of sealing products for use within their medical devices.For more on our full range of O-rings see this page: HERERead more about our Life Sciences & Medical expertise: HERE

Silicone O-rings for De Soutter EcoPulse™ lavage system 
Why use Push-in-Place gaskets?Why use Push-in-Place gaskets? Where a seal groove follows an irregular path or profile, a common sealing solution is to design a custom Push-In-Place (PIP) gasket. This will have the same profile as the centre line of the groove, and simply drops into place, retained by the features of its own design. Gasket sealing overview There are many ways to seal the static join between two components. This could be to keep fluids inside a cavity, or to keep fluids or contaminants out of a device or assembly. The options will vary from simple O-rings, moulded elastomer gaskets and flat sheet style materials, to liquid gaskets (or RTV’s).As with all sealing applications, the optimal sealing solution is designed by first reviewing the application conditions. These include temperature, pressure, fluid exposure etc. Other variables such as life requirement, equipment serviceability and seal compression set will all be considered. Arguably though, compared to other sealing applications there are considerations when designing face, cover or flange sealing solutions. It is imperative to consider the packaging requirements and assembly issues of gasket sealing options. For example, if there is a need to avoid or seal around bolt holes (or other retaining/clamping devices). Additionally, consideration around optimizing hardware wall sections or depths can play an important part in choosing the most suitable gasket sealing technique. What are Push-in-Place gaskets? With the right combination of application conditions, an O-ring style approach to sealing may be the most appropriate. O-rings tend to require relatively shallow grooves compared to their cross section in one half of the assembly. In cases where the groove is round in plan view – they can be a good solution.However, in cases where the groove follows a more irregular path or profile (frequently referred to as a “racetrack”) the O-ring can sometimes pop out in places. This will be often where the two housing parts are being brought together. A common solution is designing a custom moulding with the same profile as the centre line of the racetrack groove. This will simply drop into place.A similar approach is used when the application or hardware constraints steer the design towards a gasket that has a greater cross section depth compared to the width. This would typically be designed so the centre line of the gasket matches the centre line of the groove plan profile – again so that it drops easily into place.An inherent problem with gaskets that can drop into place is that often, they easily drop out of place too. This can occur when the component needs to be inverted or has the potential for rough handling during assembly. Consequently, the gasket may become partially or fully dislodged from the groove, which results in a badly sealed interface. The best solution to this issue is to incorporate retention pips or bumps in the gasket design. This is a solution known as Push-In-Place (PIP) gaskets. These require a distinct force to put them into the groove. Subsequently, they require more than just gravity to get them out of the groove. Why use Push-in-Place gaskets? There are other less effective solutions for tricky groove sealing, such as the use of a sticky grease, or the use of an adhesive. These can bring compatibility and health and safety issues to consider. Additionally, the risk that any contaminant could keep the gasket off the surface that it’s supposed to be sealing against. As a result, the integrity of the seal can be severely compromised.Neither of these approaches can be recommended. Instead, the use of retention pips is a safe and secure way of ensuring the gasket remains in the groove. How do retention pips work on a gasket? To determine the optimum number, size and position of the retention bumps, Finite Element Analysis is used. This ensures they provide sufficient squeeze to prevent the gasket being easily dislodged. Additionally, it’s important the groove space isn’t overfilled with seal material or interfering with the seal compression footprint against the hardware faces.The bumps can be strategically positioned to control any distortion of the gasket under pressure or temperature conditions. For example, low temperature conditions can shrink the gasket and tighten the radius it adopts around a bend in the racetrack profile. This can reduce the seal compression locally and potentially create a leak path.By positioning retention bumps at either end of the bend, the thermal contraction will be controlled to minimize leakage risk.Effective retention is needed to ensure the gasket remains correctly located in the groove even with varied use. For example, if the part needs to be inverted (sometimes the preferred assembly method for practical reasons). Alternatively, it could be subject to rough handling.For large gaskets this is normally the most effective solution. On smaller gaskets (particularly those located well inside the periphery of the assembly), there is a significant risk of a dislodged gasket being totally undetected unless using a PIP gasket design. Monitoring gaskets - human eye or testing machinery It is possible to include tell-tale signs on a gasket design. For example, if a part of the elastomer gasket protrudes sideways through a gap in the housing wall the presence of the gasket can be checked. This will be either with the human eye or an automated vision system. However, this does not ensure correct seating all around the gasket length. Additionally, it cannot be used for internal gasket locations. In these cases a missing or badly fitted gasket would only be discovered during post-build testing, or even worse with a machine failure at a customer.If included at the design stage, the small additional tooling and material costs associated with a PIP gasket are negligible compared to the costs of an impossible assembly scenario, strip and re-build costs on the assembly line, or the consequential costs associated with failure of an assembly once delivered to a customer.More information on PIPs and gaskets can be found HERE
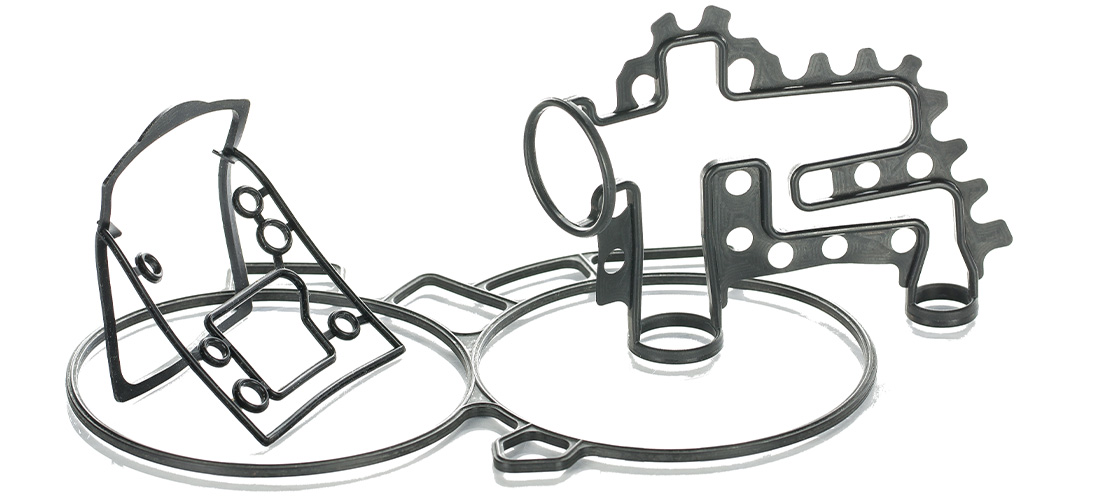
Why use Push-in-Place gaskets? 
Perfluoroelastomers in valvesPerfluoroelastomers in valves Is it time to re-visit using perfluoroelastomer seals in your valves? First developed by DuPont™ in the late 1960s, perfluoroelastomers (or FFKMs), are now widely known and understood in a variety of markets. But for those that may be less familiar with these high performance materials, here is a quick recap... What are perfluoroelastomers (FFKMs)? They are essentially highly or fully fluorinated compounds with a fluorine content above 75%. They offer outstanding chemical resistance - generally better than all other elastomer types. FFKMs are often referred to as having the resistance of PTFE but in elastomer form. The term “universal” chemical resistance is commonly used; although it’s not strictly true as we will learn shortly.Unlike PTFE, the molecular make-up of FFKM includes crosslinks (or spring-like elements). This is instead of just a backbone of carbon-carbon atoms surrounded by protective fluorine atoms. These crosslinks give the FFKMs their crucial elastic behaviour (i.e. returning quickly to their original shape after being deformed). However, the crosslinks are also a drawback as they can be a weak point for a chemical attack.Different crosslinking systems can be used when developing FFKMs and the choice will determine the high and low-temperature capabilities. Compounds developed for extreme high temperatures (up to around 325°C) generally have a less broad chemical resistance compared to the lower temperature grades (up to 225°C). Similarly, FFKMs developed to have excellent resistance to specific fluids (such as amines or high-temperature steam) can have limitations of low-temperature capability or compression set. As a result, there is no universal material that covers all application criteria bases. A variety of grades Previously, the number of perfluoroelastomer grades was less prolific than other elastomer types such as FKM, EPDM, and NBR. However, over 50 years of technical developments have created a range of FFKM grades for specific and challenging applications. These applications are particularly in chemical process, oil and gas, semiconductor, and aerospace industries. The portfolio of FFKM-based compounds available to engineers is now substantial. This is due to many options being available, which include FFKMs with a hardness range of 65 to 90 durometer. Additionally, versions that meet international standards or specifications for food, medical, CPI, and oil & gas applications also being available.In addition to technical developments, manufacturers and compounders have been addressing the only real drawback of FFKM materials; the cost. They are difficult and time-consuming base polymers to manufacture. Coupled with a relatively low volume production base and sometimes lengthy processing times for seal manufacture, FFKM seals have always carried a high financial premium over FKM seals. Even several times greater than FKM itself has over NBR.Recently, there’s been more focus on making general-purpose grade FFKMs with broader temperature and chemical resistance capabilities more financially attainable. The initial procurement costs remain high compared to less capable elastomer bases. However, the overall cost of ownership may now be more appealing than it was twenty or even ten years ago. It is possible for FFKM seals to survive for much longer in applications where exposure to a variety of fluids (perhaps wider than originally specified). This considerably reduces unplanned costs associated with maintenance and downtime. Focus on dry coatings The cost of unscheduled maintenance and repair in pump and valve equipment can be high in any industry. It is exceptionally so in petrochemical, oil & gas, and semiconductor. When these costs are fully considered in the overall lifetime of a product, the initial price of seals in a valve is considered relatively minor. However, it can still be a barrier in the material selection process.With both technical and commercial developments in recent times, FFKM materials now compare more favourably against other materials. These include static, or low-duty dynamic applications in valves. In applications where persistent and sporadic issues keep coming back to cause problems, they are now a more financially attainable choice of material to avoid re-work, overhaul, downtime, customer dissatisfaction, and ultimately, more costs.Read more about FFKM as a suitable sealing material HERE

Perfluoroelastomers in valves 
Seals for Life Sciences & Medical ApplicationsSeals for Life Sciences & Medical Applications When designing and manufacturing sealing solutions for applications within critical devices and equipment, the strictest demands in product integrity and the highest specifications of hygiene and cleanliness must be met.The Life Sciences & Medical industry is one of the most demanding and stringently regulated. There are a variety of areas of product development and manufacture where seals for Life Sciences & Medical Applications are required. Diagnostics Technology changes over recent years mean there’s now many ways of supporting patients with accurate results. This means there can be safe management of health conditions; both at home and within a clinical setting. Either way, it’s vital that diagnostic devices and systems are developed with accuracy and speed of response. This is to enable targeted analysis and therapy. Typical applications include seals for in-vitro devices, analytical laboratory equipment, x-ray, and CT/MRI scanning equipment. We support your engineers with efficient design and project management of optimised sealing components for both clinical and patient self-diagnosis. Patient management and care For obvious reasons, repeatable and reliable control of the equipment used in patient management and care is paramount. Typical applications include seals for ventilators, anaesthesia pumps, respiratory therapy, monitoring equipment, minimally invasive surgery equipment and metered dose aerosols (such as inhalers). The typical demands on these types of seals include optimised working friction and wear life, critical feature’s function & tolerance control, and the management of gas, liquids, or solid media at accurate rates. Biotech and pharmaceutical processing Due to the continued development of complex and expensive drugs and research control media, the demand for high performance interactive components within the biotechnology process industry is crucial. Typical applications include seals for analytical equipment, pumps, valves & actuators, monitoring & control equipment and storage equipment & transportation vessels. We will support engineers on recommendations for Ultra High Purity (UHP) materials. This is for extreme applications combined with sensitive chemical media. Additionally to these elements, seal design recommendations for dead space and entrapment elimination within these applications. Ultra High Purity (UHP) materials Silicone based rubbers are a common choice of elastomer materials for seals used within medical devices. This is due to their biocompatibility features and resistance to bacterial growth. Commonly they're platinum cured for injection moulded liquid silicone rubbers (LSR's) and platinum and peroxide cured for compression moulded heat cured silicones (HCR's). There's a diverse range of silicone rubbers currently available in varying shore hardnesses. These are already USP Class VI or ISO 10993 approved, making them a go to choice if the specification allows.Sometimes, an application requires a seal material to have better mechanical properties, more versatility with temperature range or media compatibility. In this case, then EPDM and FKM rubbers are also a good choice.Many of our materials are fully compliant and have the required industry approvals, such as with FDA, USP Class VI, ISO 10993, UHP, BAM and BfR standards. Cleanroom manufacturing A controlled cleanroom environment is essential for manufacturing and packaging of products that must not have contact or contamination from external particulates. These include dust, dirt, airborne microbes, and aerosol particles.It’s created and maintained by removing the air. It’s then circulated through a filtering system, and the clean and filtered air is distributed back into the cleanroom environment. This can be achieved at varying levels of cleanliness. It also depends on what’s specified for each individual manufacturing environment or the finished product requirements.Different cleanliness levels are classified by the concentration of airborne particles within a measured space. We provide a complete cleanroom production process. From material blank production through to inspection and packaging using controlled materials within state-of-the-art cleanrooms.Read more about our Life Sciences & Medical industry expertise HERE

Seals for Life Sciences & Medical Applications 
3D printing for seals3D printing for seals 3D printing has developed significantly and now performs a crucial role in many applications. 3D printed products vary from fully functional to purely aesthetic applications – commonly for manufacturing. Here we discuss how our engineers use this technology to demonstrate a seal concept. What is 3D printing? The typically common name for additive manufacturing is 3D printing. The process involves the construction of a three-dimensional shape designed and generated from a computer aided design program (or CAD). The most typical process used for 3D printing is FFF (Fused Filament Fabrication) or FDM (Fused Deposition Modelling).The FDM process uses a continuous filament of a thermoplastic material. It’s then deposited onto the 3D print bed, creating layer by layer, gradually building up the 3D model structure. We commonly use this process to create our design and development range of 3D printed models and seal prototypes. What material is suitable for 3D printing? Material compatibility with the process is imperative and a range of thermoplastic grades are used. Typical suitable material grades include; Polylactic acid (PLA), Acrylonitrile Butadiene Styrene (ABS), Thermoplastic Polyurethane (TPU), Nylon and Polypropylene (PP).The most common grade we use for 3D printing is PLA – for its great strength and stability. We’ve also adapted our design process to use more TPU based material grades, as it loosely demonstrates the same properties as elastomer grades and is better for using in prototype programmes where mechanical fit in groove is tested. Why use 3D printing for seals? 3D printing is used for a variety of designs and seal types. From O-rings, gaskets and lip seals – to grommets, multi shot mouldings and large seal assemblies.Using 3D printing during the initial design stages of a project comes with many benefits. The rapid turnaround means a simple seal design will be produced in around 15 minutes. Additionally, even more complicated parts can be manufactured in the same day. We can even print the application housings – a perfect demonstration to an engineer what they can expect from a seal part. They will see the shape and fit for hardware without the lead time and cost of cutting a prototype tool for moulded parts.This solution is particularly suitable for quick turnaround gasket designs. For example for automotive applications or similar critical markets. Our engineers can design the concept and then 3D print a rapid prototype of a gasket to suit a 3D printed gauge groove. Demonstrating to our customers that the seal has been fit checked for installation, builds further confidence in the design recommendation. Our engineers combine the 3D print with FEA simulation reports to offer a fully engineered sealing solution.Learn more about our design and simulation service HERE
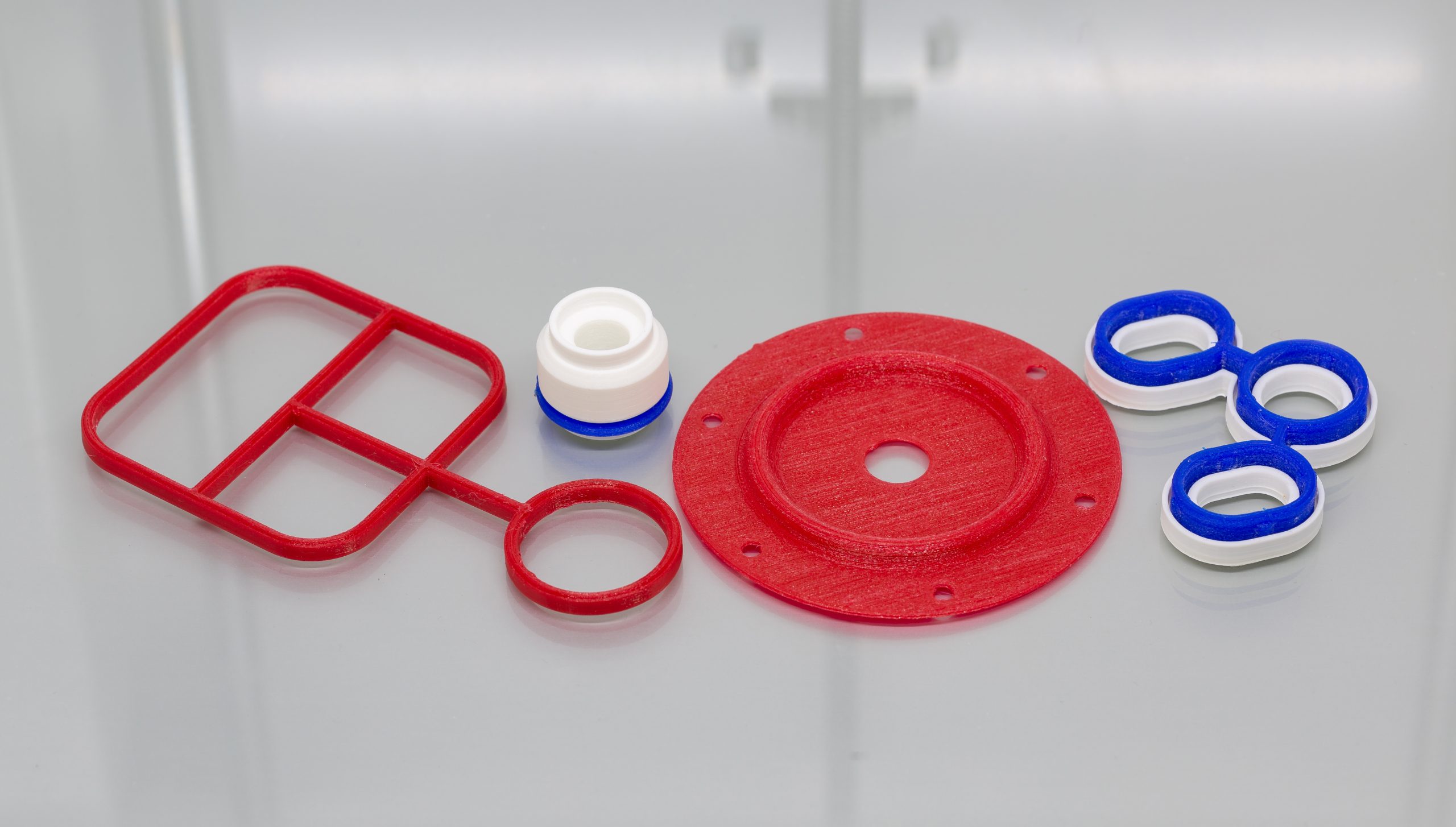
3D printing for seals 
E1244-70 seals in Life Sciences & Medical applicationsE1244-70 seals in Life Sciences & Medical applications Packed with multiple benefits, E1244-70 recommended for seals in Life Sciences & Medical applications. Specifically suitable for pharmaceutical manufacturing, biopharmaceutical processing and disposable medical devices, E1244-70 is an internally lubricated compound. With this material, there is no need for an external lubricant. What is E1244-70? E1244-70 is a 70 durometer internally lubricated, black EPDM (Ethylene Propylene Diene Monomer Rubber) material. Possessing an internal lubricant, which reduces installation force and dynamic friction, E1244-70 is compatible with all water-soluble chemistries. Additionally, it has excellent resistance to repeated conditions such as steam, gamma, ozone and ethylene oxide sterilization. Adding external lubricants can be problematic due to the risk of leaking into flow paths and migrating into areas where they are not needed. Additionally, even ‘clean’ lubricants like USP silicone, which can trap dirt and dust, can compromise patient health. Removing the need for an external lubricant by using E1244-70 is clean and safe with no risk of leakage. Benefits, application and use With a temperature range of -54°C to 121°C (-65 to 250°F), E1244-70 has a low compression set, and is suitable for both dynamic and static seal applications. Because this material does not need an additional lubricant, it prevents multiple issues associated with non-lubricated seals. For example, mismanagement by not using a lubricant when needed, can lead to friction and heat build-up, resulting in erosion and potential leakage and failure of the application. The material is compliant with USP Class VI biocompatibility and USP cytotoxicity standards (for life sciences applications). The internal lubricant derives from the fatty acid family, which significantly reduces patient reactions. This means it is safe for medical devices and pharmaceutical applications. In summary, it is suitable for:Dynamic applications & difficult installationsSurgical instrumentsPharmaceutical manufacturingBiopharmaceutical processingDisposable medical devicesRepeated device sterilization Our Life Sciences & Medical expertise We have clean room manufacturing facilities which are Class 7 (10,000) manufacturing and Class 5 (100) inspection, cleaning, and packaging.Our application engineers utilise the latest in 2D/3D CAD and FEA simulation software to design and replicate seal performance. This is before finalising each individual seal design, incorporating significant feature and critical function elements for integration with customer mating parts.We offer material development and testing, and a component endurance testing service.Learn more about how we support our customers in the Life Science and Medical Industries HERE

E1244-70 seals in Life Sciences & Medical applications